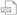Flexible Container
AVAILABILITY*
Image filed
UoS† NDC
CONCENTRATION
CONTENT
PRODUCT INFORMATION‡§
PRODUCT RESOURCES
ALERT
Supply Available

00409-0152-24
5 mg/mL
500 mg/100 mL
Non-DEHP
Latex-Information‡
Preservative-Free§
Cap Color:
N/A
Product Numbers
UNIT OF USE NDC:
00409-0152-01

UNIT OF SALE NDC:
00409-0152-24

Ordering Information
ORDER SIZE:
1 Case of 24 Bags
CASE SIZE:
1 x24
CASES PER PALLET:
96
Unit of Sale Weight:
7.8
Unit of Sale per Shipper:
96
Shipper Outer Dimensions:
11
Shipper Weight:
7.8
Wholesaler Information
ABC:
10263813
Cardinal:
5760814
McKesson:
2379006
Morris & Dickson:
144287
Availability Specifics
Status:
Supply Available
Additional Information
View
Depleted

00409-7811-37
5 mg/mL
500 mg/100 mL (Quad Pack)
Latex-Information‡
Preservative-Free§

Cap Color:
N/A
Product Numbers
UNIT OF USE NDC:
00409-7811-11

UNIT OF SALE NDC:
00409-7811-37

Ordering Information
ORDER SIZE:
1 Case in Quad Pack Overwraps (80 Flexible Containers)
CASE SIZE:
1 x 80
CASES PER PALLET:
36
Wholesaler Information
ABC:
10015180
Cardinal:
1668532
H. D. Smith:
1236611
McKesson:
1251701
Morris & Dickson:
133371
Availability Specifics
Status:
Depleted
Delivery Date:
12/2027
Date product is expected to arrive at Pfizer’s US product distribution centers. Orders will release as deliveries arrive. All orders filled by estimated recovery date.
Estimated Recovery Date:
4Q 2027
Date Pfizer believes supply will return to uninterrupted supply levels.
Back order Reason:
Discontinuation of the manufacture of the drug
Additional Information
View
Not all brand names mentioned on our site are Pfizer products.
*Availability information on the site has been updated to match the current information from the availability report.
Information on this page may change without notice.
†Unit of Sale.
‡“Latex Information” indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.
§“Preservative Information” indicates that this product does not contain ingredients identified as a preservative, as defined by the USP.
Product Numbers