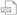Flexible Container
AVAILABILITY*
Image filed
UoS† NDC
CONCENTRATION
CONTENT
PRODUCT INFORMATION‡§
PRODUCT RESOURCES
ALERT
Depleted
00409-4883-10
2 mg/mL
600 mg/300 mL
Non-DEHP
Latex-Information‡
Gluten Free
Cap Color:
N/A
Product Numbers
UNIT OF USE NDC:
00409-4883-03

UNIT OF SALE NDC:
00409-4883-10

Ordering Information
ORDER SIZE:
1 case of 10 containers
CASE SIZE:
1 x 10
CASES PER PALLET:
90
Unit of Sale Dimensions:
15.20 x 7.70 x 6.40
Unit of Sale Weight:
7.963
Shipper Outer Dimensions:
15.20 x 7.70 x 6.40
Shipper Weight:
7.963
Wholesaler Information
ABC:
10280845
Cardinal:
5855887
McKesson:
2830271
Morris & Dickson:
290981
Availability Specifics
Status:
Depleted
Delivery Date:
06/2025
Date product is expected to arrive at Pfizer’s US product distribution centers. Orders will release as deliveries arrive. All orders filled by estimated recovery date.
Estimated Recovery Date:
3Q 2025
Date Pfizer believes supply will return to uninterrupted supply levels.
Back order Reason:
Manufacturing Delay
Additional Information
View
VisIV™ Flexible Container
AVAILABILITY*
Image filed
UoS† NDC
CONCENTRATION
CONTENT
PRODUCT INFORMATION‡§
PRODUCT RESOURCES
ALERT
Depleted

00409-4883-01
2 mg/mL
600 mg/300 mL
Non-DEHP
Latex-Information‡
Gluten Free
Cap Color:
N/A
Product Numbers
UNIT OF USE NDC:
00409-4883-11
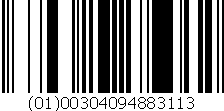
UNIT OF SALE NDC:
00409-4883-01
Ordering Information
ORDER SIZE:
1 Case (10 Flexible Containers)
CASE SIZE:
1 x 10
CASES PER PALLET:
45
Wholesaler Information
ABC:
10150966
Cardinal:
5089776
H. D. Smith:
5470703
McKesson:
3451150
Morris & Dickson:
232926
Availability Specifics
Status:
Depleted
Delivery Date:
12/2027
Date product is expected to arrive at Pfizer’s US product distribution centers. Orders will release as deliveries arrive. All orders filled by estimated recovery date.
Estimated Recovery Date:
4Q 2027
Date Pfizer believes supply will return to uninterrupted supply levels.
Back order Reason:
Manufacturing Delay
Additional Information
View
Not all brand names mentioned on our site are Pfizer products.
*Availability information on the site has been updated to match the current information from the availability report.
Information on this page may change without notice.
†Unit of Sale.
‡“Latex Information” indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.
§“Preservative Information” indicates that this product does not contain ingredients identified as a preservative, as defined by the USP.
Product Numbers